
HPFB's Mandate is to take an integrated approach to the management of the risks and benefits to health related to health products and food by: Our mission is to help the people of Canada maintainand improve their health. Published by authority of the Minister of Health Should you have any questions regarding the content of the guidance, please contactīureau of Metabolism, Oncology and Reproductive Sciences,įax: 61 Guidance Document Basic Product Monograph Information for Nonsteroidal Anti-Inflammatory Drugs () This and other Guidance documents are available on the website.
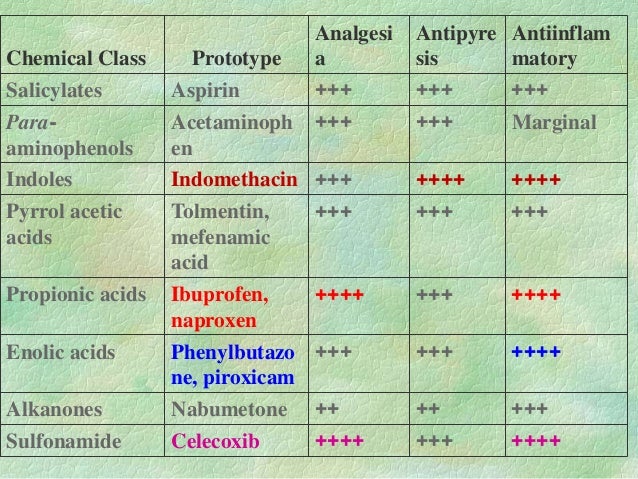
#Nsaid cross reactivity chart update#
All new will be required to be in complete compliance with the guidance.ĭepending on the market status of the Canadian Reference Product (CRP), sponsors of generic products may be permitted to update the labelling of their respective products immediately upon the finalization of the labelling for the CRP.All other sponsors will be required, with any subsequent filing, to revise the labelling of their NSAID products in accordance with the complete guidance.At the time of this posting these products include, but are not limited to: Sponsors of products belonging to the sub-group of COX-2s, products that exhibit COX-2 properties, and NSAID products of the greatest use in Canada, will be contacted by Health Canada and requested to update to the complete guidance.Sponsors will be required to revise their labelling in accordance with the complete guidance through the provision of a supplemental New Drug Submission as follows: This guidance is intended to help the pharmaceutical industry revise the content of the Product Monograph and associated labelling materials. In light of this new safety information, that should be conveyed to health practitioners and their patients, Health Canada has developed this updated version of the previous guidance document on the same subject. Continuing post-market experience from this sub-group, and as a whole, have provided new safety information beyond that which was previously described in guidance. Since the posting of the previous guidance document on, several new products belonging to the NSAID sub-group of selective COX-2 inhibitors have been approved in Canada. This document supercedes the 1997 policy: Nonsteroidal Anti-inflammatory Group of Drugs (), Revision of the General Basic Guidelines for Product Monographs of and Basic Minimum Information for the Patient Taking. Comments and suggestions received from the consultation on the draft version of the guidance were reviewed and considered in the finalization of this document. The final version of this Health Canada guidance document: Basic Product Monograph Information for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) is now available. Contact: Bureau of Metabolism, Oncology and Reproductive Sciences (BMORS) Notice


 0 kommentar(er)
0 kommentar(er)
